Making her own history: Clemson history alumna patents medical device
By Jordan Sims, Clemson University

Jamie Shultz’s ’12 Clemson degree set her up for success as a promising, young attorney at King and Spalding LLP in Atlanta. But when her health began to decline, her Clemson values proved to go far beyond a career. Shultz now owns a patent for her first-on-the-market medical device that was born out of her personal health struggles and is helping kids and adults across the country.
Clemson Beginnings
Originally from St. Louis, Shultz knew that she wanted to go to Clemson before her campus tour even started.
“No where else I had been to had that sense of home,” she said. “The kindness of the people, the way the students were interacting with one another. It was an academic setting, but there were unique features to it outside of that.”
Even from the edge of campus, there was a balanced social-academic atmosphere at Clemson that Shultz noticed and wanted to be a part of.
After beginning in another major, she switched to history when she realized her passion to follow in the footsteps of her aunt, a well-respected United States appellate judge. Shultz quickly found a home in the Department of History and Geography.
“History was the perfect major for me,” she said. “I really liked that it opened the door to so many different opportunities,” especially as an aspiring lawyer.
“It taught me to take the facts and apply them to the rules of law,” she added.
Clemson graduation brought a wealth of good news for Shultz. She passed the bar exam, got engaged, and started her dream job at King and Spalding. That’s when the persistent chest pain started.
Her Own Health Advocate
“They said I was probably anxious and overworked,” she recalled. “I was in the hospital pretty much every weekend the rest of that year.”
Her search for answers led to even more questions. Shultz was diagnosed with multiple autoimmune diseases and the path forward seemed anything but clear. What made the journey even more difficult were the nuanced rules and regulations around the medications she was able to get. Her medical team identified a drug (IVIG) that would aid quality of life for Shultz, but the process to get the medication was continually appealed. For some, this answer would be another closed door. Shultz refused to accept that.
“When you’re chronically ill you learn — and I can attribute this to Clemson — to be your own advocate. So I asked my doctor if I wrote my own appeal if she would edit it. I did that. She made a few modifications, sent it in, and I got that infusion in September after the insurance company finally granted my appeal.”
Looking back, Shultz identified other ways her time at Clemson empowered her for the health journey ahead.
“Clemson was different from other universities,” said Shultz. “You were encouraged to talk to your professor if you had a question. I learned that it’s okay to ask for help and that you should ask questions. Those things have been really helpful tools as I’m navigating my health.”
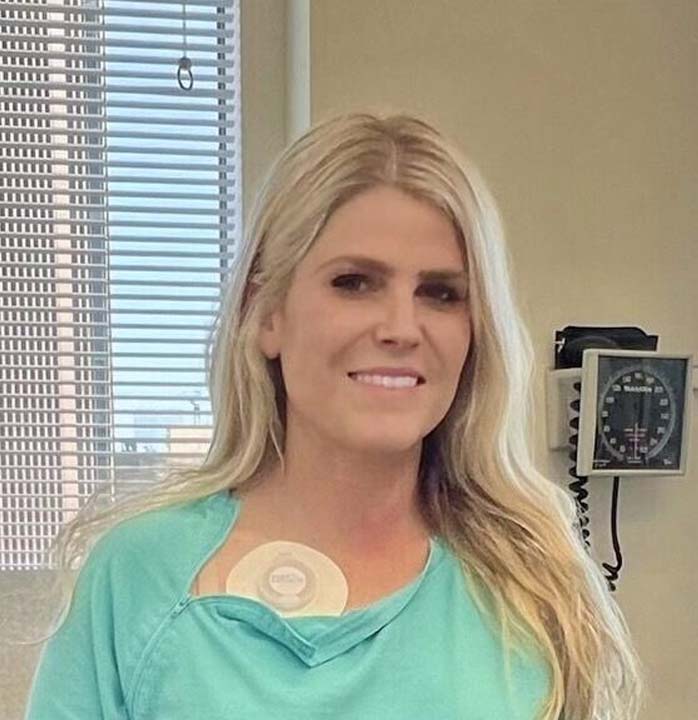
With the IVIG available, Shultz transitioned to receiving medications through a chest port, a small, implanted medical device that helps administer medication by allowing easy access to large veins. Her chest port was a positive step in her health journey, but the process of administering the medication was still unnecessarily painful and complex.
When preparing to receive medicine through a chest port, patients are encouraged to place a dollop of EMLA cream on the infusion site to numb the area before infusion. Though seemingly simple, it was difficult to keep the cream in place because it had to be secured by something that wouldn’t absorb it. Doctors recommended plastic wrap.
“It was terrible,” Shultz said. “I would get to the doctor’s office and the cream would be in my hair, all over my seatbelt, or completely moved across my chest. Then you weren’t even numb when they inserted the needle into the port.”
From Pain to ‘Port Protect’
Shultz scoured the internet to find a more effective option than plastic wrap. “I started looking to see if there was anything that could help, but there was nothing on the market. So I started researching because that’s what I was taught at Clemson, to figure it out. I didn’t know where to begin, so I started Googling.”
Over the next few months, she researched everything: how to create a product, what adhesives to use for sensitive skin, how to get a patent, designs that work for those with accessibility issues, how to find a manufacturer, and much more. As prototypes came together, Shultz would test the product and design herself, thinking through what was working and what wasn’t for people of varying abilities. One of the largest hurdles of the process was finding a manufacturer for the design, once a prototype had been solidified.
“When you’re a nobody and sick, it’s really hard to get people to talk to you,” she explained.
Still, Shultz did not give up on her product and eventually found a manufacturer in Georgia with a personal connection to her story. The Port Protect went into production in 2023 and Shultz just finished the patent process in 2024, making Port Protect her own.
A Legacy of Helping Others
For Shultz, Port Protect was never about making money.
“As exciting as it is to get sales and to see a business grow, there’s more behind it,” Shultz explained, as her eyes began to tear up.
“What’s really reassuring is having moms send in pictures telling me it’s the first tears-free port prep they’ve had since their kids have been diagnosed.”
Shultz has dreams beyond Port Protect, including creating a children’s book for kids, families, and siblings walking through health-related challenges, and she noted her desire to partner with nonprofits to make her product more accessible. Shultz has recently seen patients using the product as a numbing agent for other procedures as well.
From the skills and values she learned at Clemson, to the research and creation of a first-to-market product, Shultz’s mission has become clear over time.
“I could’ve always seen myself wanting to help people, but not like this,” she said. “Because nobody gets [an illness] until they get it, and nobody should ever have to get it.”
January 24, 2025
